Microscopy
Select the microscope best suited to your application
Use the interactive decision tree diagram to quickly locate details of the most appropriate microscope.
Download interactive decision tree diagram (PDF)
DMi1 Tissue Culture Microscope
Instrument capabilities
Common Applications
- Routine Bright field microscopy examination
- Designed for cell and tissue culture work
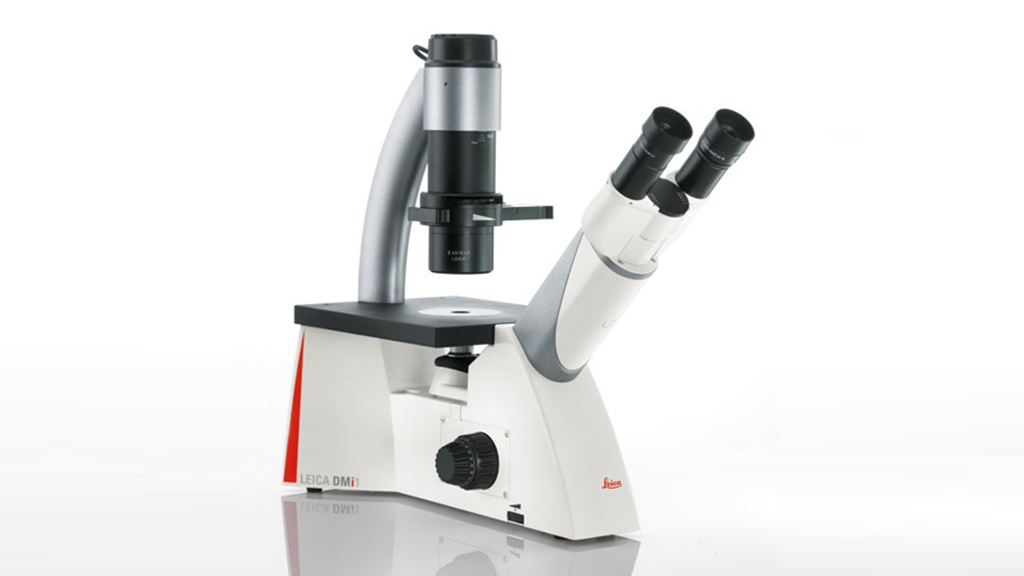
Features:
- Camera stand outfit (MC170). Capture resolution of 5 Mpixels and up to 30 fps
- 4 position slider for S40 condenser (bright field and phase contrast imaging)
- 4 Objectives (N Plan 5X, Hi Plan10, 20X, 40X)
- Object Guide
- Universal holding frame M
Software:
- Leica Application Suite (LAS)
Leica M60 Stereoscope
Instrument capabilities
Common Applications:
- Sample preparation
- Carry out close work such as dissection, microsurgery
- Viewing insects, crystals, plant life, circuit boards etc.
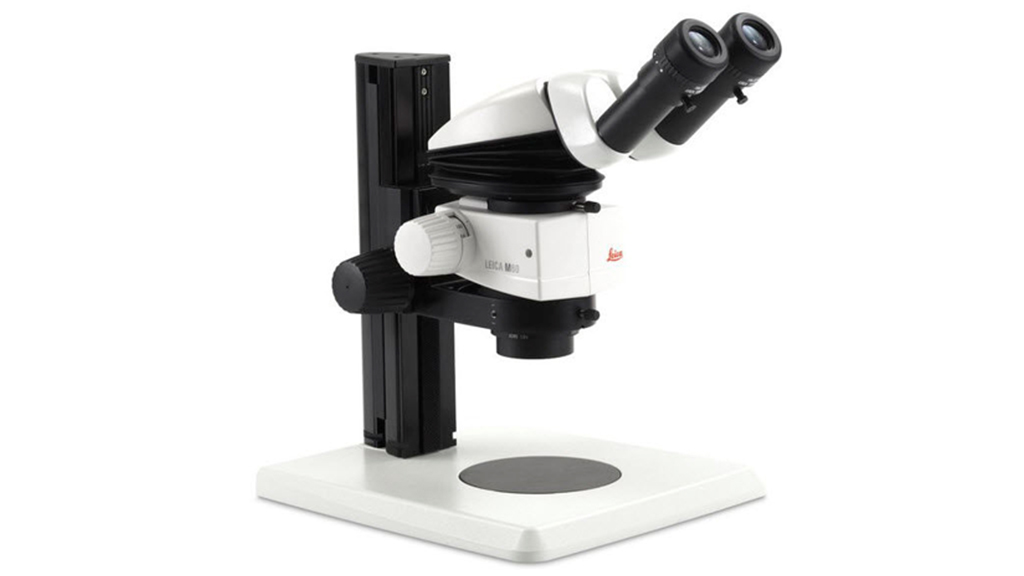
Features:
- Zoom ratio: 6:1
- Magnification range: 6.3x - 40x (1x objective, 10x eyepieces)
- Resolution up to 225 lp/mm (1x objective, 10x eyepieces)
- Transmitted light base TL5000, bightfield and two sided darkfield
- Coarse Focus drive, column 300mm
- Objective achromat 1.0x, WD 90mm
PAULA (Personalised Automated Lab Assistant)
Instrument capabilities
Common Applications:
- PAULA is a smart digital imager designed for cell culture lab
- Transfection efficiency, cell counting, confluence check and wound healing work

Features:
- Alignment free Phase contrast, red and green (emission) fluo LEDs
- Magnification: 10x with a 4x digital zoom
- Illumination: LED
- An integrated barcode reader can scan and identify culture flasks quickly and reliably
Software:
- PAULA Software includes image and t-lapse acquisition, data and user management
- Controlled with a touch monitor and a NUC PC (iOS, Android and Windows)
DM IL LED Inverted Fluorescence Microscope
Instrument capabilities
Common Applications:
- Ideal for fluorescence and brightfield laboratory cell analysis
- Image 2D cell cultures (monolayer Adherent best)
- Tissue sections

Features:
- DM IL includes LED Illumination for transmitted light
- CoolLED with Filter sets: DAPI /FITC / TXR (mCherry)/ Y5
- Phase Contrast and Fluorescence NPLAN objectives: 5x/0.12 PH, 10x/0.25 PH, 20x/0.35 PH, 40X/0.55 PH
- Condenser S 40 / 0.45
- Object guide
- Universal holding frame M
- Trinocular tube HC ILT, with viewing angle 45° beamsplitter 0/100%, 100/0%
- Leica DFC3000 G digital high-sensitivity monochrome camera. CCD technology 1.3 MPixel with a pixel size of 3.75 μm.
Software:
- Leica Application Suite X (LAS X)
Stereoscope THUNDER Imager Model Organism Automated
Instrument capabilities
Common Applications:
- Imaging of organism used for developmental or molecular biology research, such as Drosophila, C. elegans, zebrafish, etc.
- 3D tissue imaging
- Suited for demanding high-speed fluorescence applications
- THUNDER Imager delivers blur-free images revealing the fine structural details of live organisms, while keeping them under optimal physiological conditions.

Features:
- Leica M205 FA optics carrier fully motorized with: magnification, three position fluorescence filter turrent, fluorescence light shutter, and Iris diaphragm
- Zoom-magnification changer 20.5:1
- Magnification area: 7.8x-160x
- Resolution up to 525 lp/mm (1x objective, 10x eyepieces), maximum resolution up to 1050 lp/mm (2x objective, 10x eyepieces).
- Motor focus drive short 420mm
- Triple Beam TM: Separate optical beam path for fluorescence illumination
- SmartTouch TM control unit
- White LED light source for fluorescence (Lumencor SOLA-SMII (365nm Version).
- Wavelength range: 350 – 680 nm CFP/GFP/mCherry
- Motorized XY-Scanning stage (Leica LMT260)
- Leica DFC9000 GTC camera:
- Is a deep-cooled 4.2 MP sCMOS camera with a maximum of 82% quantum efficiency
- ~90 frames per second acquisition speed, and extreme low dark current of 0.14e-/p/sec.
- Pixel: 2048 x 2048 (4.2 Megapixel). Pixel size: 6.5 μm
Software:
- Leica Application Suite X (LAS X)
- LAS X Navigator
- THUNDER Imager offers three exclusive computational clearing modes:
- Instant Computational Clearing (ICC) for instantaneous removal of out of focus background
- Small Volume Computational Clearing (SVCC) dedicated to thin specimen
- Large Volume Computational Clearing (LVCC) dedicated to thick specimen
DMi8 THUNDER Inverted 3d Live Cell Fluorescence Microscope
Instrument capabilities
Common Applications:
- THUNDER Imager is designed for imaging of cell culture assays
- Imaging of 3D cell cultures and tissue, cell migration, tumors, spheroids, neurospheres, organoids, etc.
- Maintains optimal physiological conditions by minimizing photobleaching
- Provides high-performance imaging and high-throughput of data with a better workflow efficiency and statistics
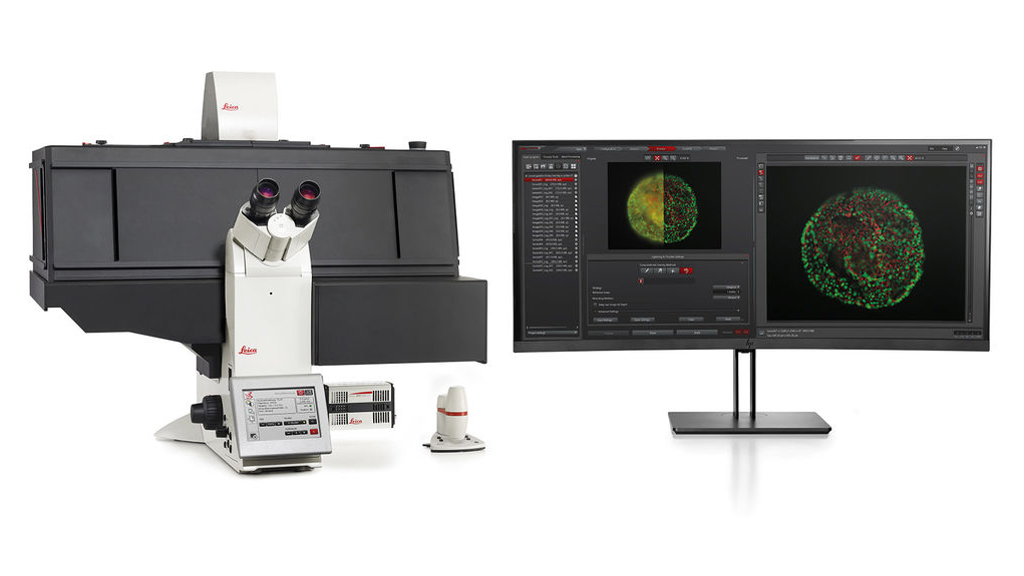
Features:
- Leica DMi8 fully motorized, inverted research stand
- Hardware based adaptive focus control (AFC) for drift correction and fast focusing in multi well applications
- Closed loop focusing system for high precision focusing with reproducibility of 20 nm
- Nosepiece, motorized 6-fold revolver
- ICT condenser prism
- Universal holding frame KM, Click-In
- Touch screen
- Leica DFC9000 GTC camera: 4.2 MP sCMOS camera with ~90 frames per second acquisition speed, and pixel size of 6.5 μm
- Incubator i8 - BLACK LS.
- Temperature controller, heating unit and CO2 controller
Light Sources and Filters:
- LED Source
- External Filter Wheel (EFW) slider for high speed acquisition – Filter set - quad cube for imaging (DAPI/405, FITC, TRITC, Cy5)
- 4 filter sets with Excitation wavelengths: 391/32, 479/33, 554/24, 638/31. Emission wavelengths: 435/30, 519/25, 594/32, 695/58. Dichroics: 415, 500, 572, 660
- -Includes filter wheel for EFW (5 positions with emission filters: 440/40, 510/40, 590/50, 700/75, 100% transmission)
Objectives:
- HC PL FLUOTAR 1.6x/0.05
- HC PL FL L 20x/0.40 CORR PH1
- HC PL APO 20x/0.80 PH2
- HC PL APO 40x/0.95 CORR
- HCX PL APO 40x/1.10 W CORR
- HC PL APO 63x/1.40-0.60 OIL
Software
- Leica Application Suite X (LAS X)
- LAS X Navigator
- LAS X 3D Analysis and Visualization
- THUNDER Imager offers three computational clearing modes:
- Instant Computational Clearing (ICC) for instantaneous removal of out of focus background
- Small Volume Computational Clearing (SVCC) dedicated to thin specimen
- Large Volume Computational Clearing (LVCC) dedicated to thick specimen
DMi8 TIRF Inverted Fluorescence (Total Internal Reflection Fluorescence)
Instrument capabilities
Overview:
Total Internal Reflection Fluorescence (TIRF) is a microscopy technique for the visualization of fluorophores that are immediately adjacent (<250 nm) to a glass coverslip. TIRF microscopes direct the excitation laser at an oblique angle to create total internal reflection that generates a highly restricted electromagnetic field (or evanescent waver) within the specimen.
Common Applications:
- To study molecular events at or near the cell surface such as cell adhesion, neurotransmitters secretion, binding of cells by hormones
- Can be used for single molecule localization research
- Tracking movement in the cell membrane, endocytosis, exocytosis, etc.

Features:
- Eyepieces HC Plan S 10x/25 Br. M
- Motorized coarse and fine focus in combination with Leica adaptive focus control (AFC)
- Scanning Stage Leica with 1mm spindle
- Universal holding frame KM, Click-In and Universal mounting frame K100
- Brightfield / Phase Contrast / Darkfield / DIC motorized
- Motorized Objective Prism Turret
- Motorized Condenser with fixed head S28 / N.A. 0.55
- Analysator Cube for motorized DIC
- ICT-condenser prism
- -TIRF Infinity Scanner
- Leica DFC9000 GTC camera: 4.2 MP sCMOS camera with ~90 frames per second acquisition speed, and pixel size of 6.5 μm
- Incubator i8 BLACK LS with laser safety
- Temperature controller, heating unit and CO2 controller
Light Sources and Filters:
- LED with TTL-Shutter
- External light source EL6000 with light guide
- Laser module 488nm (150mW) and 638nm (150mW)
- Motorized 6 -fold Filter Turret:
- Filter cubes LED 405, GFP, TXR, and LED 620
- Filter cube QWF-T. Excitation 400/28, 490/20, 561/15, 640/20, Dichroic: 422, 508, 576, 658.
Emission: 448/48, 531/32, 598/40, 705/90
Objectives:
- HC FL PLAN 5x/0.12
- HC PL FLUOTAR 10x/0.32 PH1
- HC PL APO 20x/0.80 PH2
- HC PL APO 40x/0.85 CORR CS
- HC PL APO 63x/1.40-0.60 OIL
- HC PL APO 100x/1.47 OIL CORR TIRF
Software:
- Leica Application Suite X (LAS X)
- LAS X Navigator
SP8 Confocal Microscope
Instrument capabilities
Overview:
Confocal laser scanning microscopy is one of the most popular techniques for imaging fluorophores in biological specimens. This optical imaging method increases optical resolution, contrast and in-focused images by removing out of focus light during image collection. This is achieved by using a spatial pinhole. Confocal specimens are illuminated by a focused point of laser light that is scanned across the specimen in the horizontal plane. Capturing multiple 2D images at different depths enables the reconstruction of 3D structures within a sample.
Common Applications:
- Powerful microscope (workhorse) suited to high quality imaging of fixed and live cells through time
- Ideal for 3-D imaging, time course experiments, real time visualisation of rapid cell responses
- Delivers high resolution required for multi-dimensional observation of cell and tissue morphology as well as precise molecular localisation

Features:
- Leica TCS SP8 confocal scanning microscope
- Closed Loop Focus with Adaptive Focus Control (AFC)
- Spill protection for DMi8
- Optical Outfit + motorized ICT
- Scanning stage inverted
- Universal holding frame K
- Condenser prisms for transmitted light interference contrast
- Tandem scanner 12 kHz
- Switchable resonant and non-resonant (1 -1800 Hz) mode for optimal scanning speeds
- Ludin Environmental Chamber (gas and temperature controller)
- Camera sCMOS Hamamatsu W-View Gemini provides excellent transmittance across a broad range of wavelength (40nm to 800nm). Simmultaneous image acquision of dual wavelength images. Resolution of up to ~2000 pixels x 1000 pixels per image
Light Sources and Filters:
- External light source EL6000
- Laser 405 nm DMOD Compact, Laser blue 488 nm, Green 552 nm, Red 638 nm
- Two internal detector channels (PMT), one internal detector channel HyD (SP GaAsP-Detector)
- Transmitted Light Brightfield Detector
- Filter cube DA/FI/TX
Objectives:
- HC PL FLUOTAR 20x/0.55
- HC PL APO 40x/1.30 OIL CS2
- HC PL APO 63x/1.40 OIL CS2
- HC PL APO 93x/1.30 GLYC motC STED W
Software and modules available:
- Leica Application Suite X (LAS X)
- LAS X Navigator
- LAS X 3D Visualisation
- Huygens Base package for confocal (De-convolution)
SP8 Advanced Confocal FALCON (White Light Laser – FAst Lifetime CONtrast)
Instrument capabilities
Overview:
SP8 FALCON (FAst Lifetime CONtrast) a powerful microscope equipped with a truly integrated solution for Fluorescence Lifetime Imaging (FLIM), Fluorescence Resonance Energy Transfer (FRET) and Fluorescence Correlation Spectroscopy (FCS) to investigate cellular physiology and explore dynamics in living cells.
- The FLIM approach on SP8 FALCON is based on a confocal scan head with field-programmable gate array (FPGA) electronics, pulsed laser excitation and fast, spectral single-photon counting detectors
- Fluorescence Resonance Energy Transfer (FRET) is an optical technique used to detect molecular interactions and requires specific donor and acceptor fluorophore pairs. Measurements of FRET efficiency can be used to determine if two fluorophore are within a certain distance of each other
- Fluorescence Correlation Spectroscopy (FCS) is a technique that allows the measurement of diffusion coefficients and concentrations for fluorescing constructs
Common Applications:
- The applications of FLIM are many: from ion imaging and oxygen imaging to studying cell function and cell disease.
- FRET is a useful tool for quantifying protein-protein interactions, protein–DNA interactions, protein conformational changes, and to detect the location and interactions of genes and cellular structures including intergrins and membrane proteins
- FCS and FLCS can measure any fluorescently labelled molecules in solution (usually water or buffer solution), on membrane and even inside living cells

Features:
- Leica TCS SP8 Flexible Supply Unit White Light Laser, DMi8 CS Bino
- Closed Loop Focus with Adaptive Focus Control (AFC)
- Turret cooling for DMi8
- Spill protection for DMi8
- Optical Outfit + motorized ICT
- Scanning stage inverted
- Super Z Galvo stage type H
- Ludin Environmental Chamber (gas and temperature controller)
- Tandem scanner 8 kHz
- Leica DFC7000 T Camera: The state-of-the-art CCD sensor provides quality and speed to reproduce colors faithfully with intelligent interpolation to match illumination settings
- Features 2.8 megapixel with a pixel size resolution of 4.54 µm, 40 fps at full resolution and 50 fps with exclusive turbo mode
Light Sources and Filters:
- External light source EL6000
- Laser 405 nm AOTF Flexible
- White light laser (WLL) for Acousto-Optical Beam Splitter (AOBS) system. Excitation lines from 470 to 670 nm.
- Pulse frequency max. 80 MHz, variable by means of pulse picker
- Filter cube DA/FI/TX and filter cube Y5
- Transmitted Light Brightfield Detector
- Four Internal Detector Channels:
- Internal Detector Channel HyD SMD 1
- Internal Detector Channel PMT 2
- Internal Detector Channel PMT 3
- Internal Detector Channel HyD SMD 4
Objectives:
- HC PL APO 10x/0.40 CS2
- HC PL APO 20x/0.75 IMM CORR CS2
- HC PL APO 40x/1.10 W CORR CS2
- HC PL APO 63x/1.40 OIL CS2
- HC PL APO 86x/1.20 W motCORR STED W
Software:
- Leica Application Suite X (LAS X)
- LAS X Navigator
- LAS X 3D Visualisation
- FALCON Fluorescence Life-time Imaging Microscopy (FLIM) with WLL
- LAS X Phasor FLIM
- FALCON enables Fluorescence Lifetime Correlation Spectroscopy (FLCS) experiments
- LAS X includes FLIM-FRET analyzer
- Confocal Super-Resolution LAS X LIGHTNING Expert
RESOLFT by Abberior
Instrument capabilities
Applications:
The Reversible Saturable Optical Fluorescence Transitions (RESOLFT) superresolution microscopy
RESOLFT stands for a general switching principle: the switching mechanism does no longer need to be purely electronically but can include switching mechanisms like for example conformational changes of a molecule. Strictly speaking, the STED method is one possible implementation of the RESOLFT concept. The approach is similar as shown for STED – a beam scanning setup with overlapping beams of the excitation and the switching beam.

Features:
- Superresolution RESOLFT channel compatible with fast switching GFP variants
- -Two fast scanning simultaneously recording confocal channels
- GFP, 488-dye optimized
- Motorized pinhole
- State-of-the-art fluorescence widefield and confocal stand (IX83)
- Proprietary, very-compact linear Quad-Scanner
- Software solution including data analysis package
Nikon Eclipse Ti2-U and Microinjection System
Instrument capabilities
Common Applications
High precision, compact micromanipulator system to simplify intracellular injection. It is capable of delivering precise nanoliter volumes of diverse compounds and biomolecules.

Features:
- Inverted microscope Eclipse Ti2-U main body
- DIC Polarizer
- Combined 3D motor-drive coarse manipulator and 3D oil-hydraulic fine manipulator
- Pneumatic Microinjector (IM-11-2)
- Electric Microinjector 240V (IM-400)
- Suction Unit for IM400 (IM-400B)
Objectives:
- CFI Plan Fluor 4x/0.13, W.D. = 17.1 mm
- CFI Plan Fluor 10x/0.30, W.D. = 16.0 mm
- CFI Super Plan Fluor ELWD 20x/0.45, W.D. = 8.2-6.9 mm
- CFI Super Plan Fluor ELWD 40x/0.6, W.D. = 3.6-2.8 mm