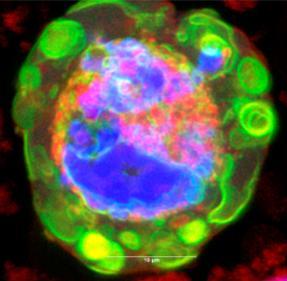
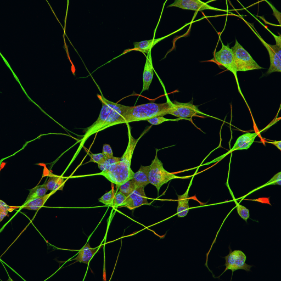
The Ecroyd research focus is in the field of protein homeostasis (proteostasis), an important area of research as disturbances in proteostasis can lead to protein aggregation (i.e. the clumping of proteins into large deposits), a pathological hallmark of many human diseases, including Alzheimer’s disease, Parkinson’s disease and Motor Neurone Disease (MND). Research in the Ecroyd lab focuses on the role of molecular chaperone proteins in proteostasis. This is because these are the body’s front-line defenders against protein aggregation. By identifying innovative approaches to activate molecular chaperones, the group aims to develop new drugs to treat, and ultimately prevent, neurodegenerative diseases such as MND.
Work currently being undertaken in this laboratory extends from molecular biology-based techniques to recombinant protein expression and purification, in vitro biochemical assays of chaperone protein activity, to mammalian cell culture and the study of protein expression and modification in animal tissues. Of late, my group has been involved in developing novel techniques to study heat-shock chaperone function in cells and a flow cytometry-based method to count and physically isolate protein inclusions from cells. The group are also developing new single-molecule approaches so that, for the first time, we can see and characterise the interactions between heat-shock proteins and aggregation-prone proteins.
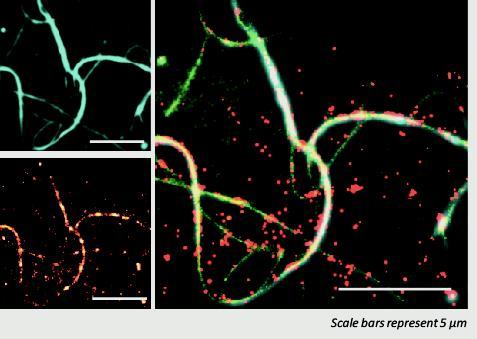
The small heat shock protein Hsp27 (HSPB1) bound to the surface of an a-synuclein amyloid fibril. This image was obtained using Total Internal Reflection Fluoresence (TIRF) microscopy. We think, by binding to amyloid fibrils, these molecular chaperones protect the cell from their toxic effects.
View Professor Heath Ecroyd's Scholars page
Contact heathe@uow.edu.au for more information.