The Immunology and Cell Signalling Group focuses on extracellular signalling pathways between immune cells in the context of health and disease in humans and companion animals. A key focus of the group is the study of signalling pathways mediated by extracellular ATP and purinergic receptors (mainly P2X7, P2X4, P2Y12 and A2A), as well ecto-enzymes (mainly CD39 and CD73), which regulate the availability of extracellular nucleotides and nucleosides (see Figure). These pathways are currently being investigated in the context of immunity, inflammation and blood clotting, as well as diseases such as cancer, graft-versus-host disease, psoriasis, inflammatory pain and motor neuron disease.
To better understand the above, the group utilises a number of technologies and approaches including flow cytometry, mass cytometry, cation flux assays, recombinant DNA techniques, mouse models including humanised mice, and blood samples from people, cats and dogs.
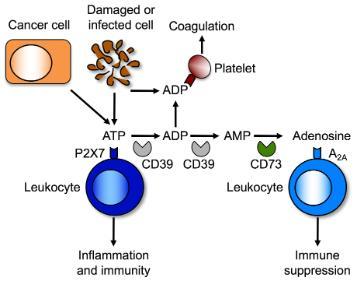
Purinergic signaling pathways amongst immune cells. ATP released from damage, infected or malignant cells can activate P2X7 (and P2X4, not shown) on leukocytes to promote inflammation and immunity. Extracellular ATP can be sequentially degraded by the ecto-enzymes CD39 and CD73 to adenosine, which can activate A2A on leukocytes to suppress inflammation and immunity. ADP released from cells or resulting from ATP degradation can activate P2Y12 on platelets to promote coagulation.
View Associate Professor Ronald Sluyters Scholar page
Contact rsluyter@uow.edu.au for more information.





