Introduction
The ozone layer has been measured regularly and globally for about fifty years since a worldwide network of stations, including the Halley Bay Station in Antarctica, was developed as part of the International Geophysical Year (1957). Since the 1970s, satellites have been widely used to observe the earth's environment, enabling fast, spatio-temporal data acquisition on a global scale. Based on an accumulation of data and science, we now have a reasonable understanding of how the ozone layer varies in the four-dimensional spatio-temporal space and of what environmental parameters are pertinent to the variation of the ozone layer. In this description, we illustrate how ozone is distributed in the atmosphere and why the ozone layer varies in the way it does; our main resource was the text, "Stratospheric Ozone: An Electronic Textbook," http://www.ccpo.odu.edu/SEES/ozone/oz_class.htm.
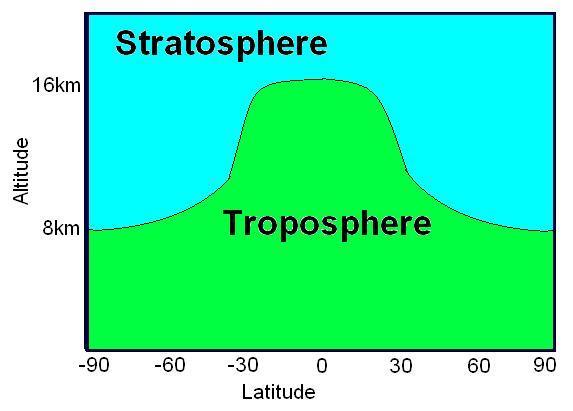
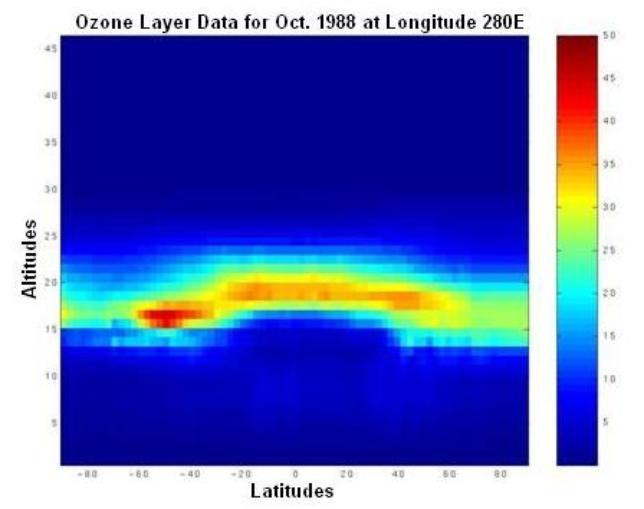
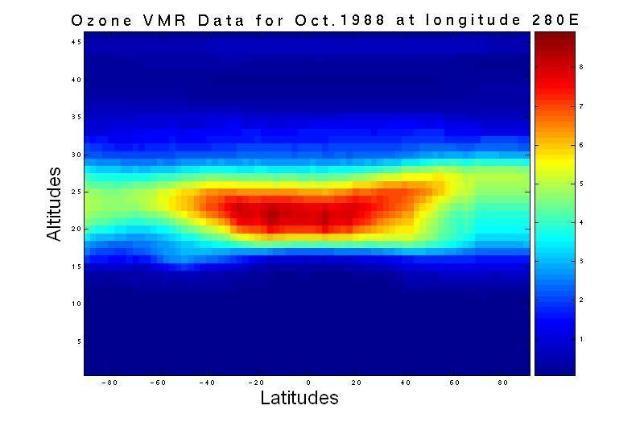
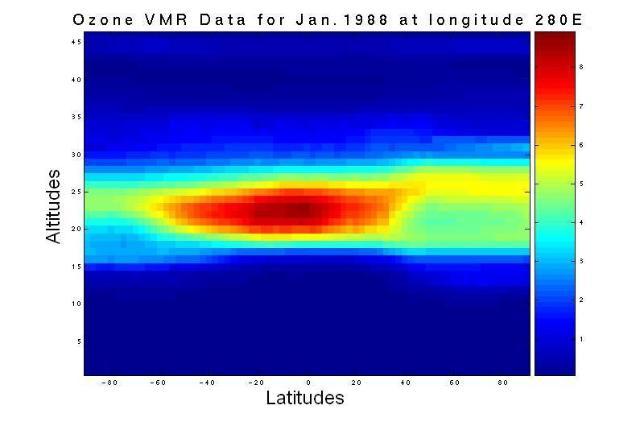
In large part, the earth's ozone distribution is controlled by the properties of the solar-earth system. First, the earth's gravity forces the air to stay around the surface of the earth and form the atmosphere. Second, the sun's energy, directly or indirectly, hits and heats the atmosphere and ground, then radiates away to outer space. Consequently, the atmosphere is stratified into several layers according to the variation in temperature. Meanwhile, UV energy from sunlight enables large-scale atmospheric chemical reactions, including the production and decomposition of ozone molecules. Due to the shape and rotation of the earth and the energy from the sun, there is variability in air pressure. Consequently, diverse air streams transport the products of the atmospheric chemical reactions globally from one place to another. Since the majority of atmospheric ozone is the result of photochemical reactions driven by the energy of the sun, the ozone distribution is dynamically and chemically determined by these processes. Figure 1 shows a plot of the two-dimensional distribution of the monthly mean total column ozone (TCO) in Dobson Units (DU) for the month of October 1988.
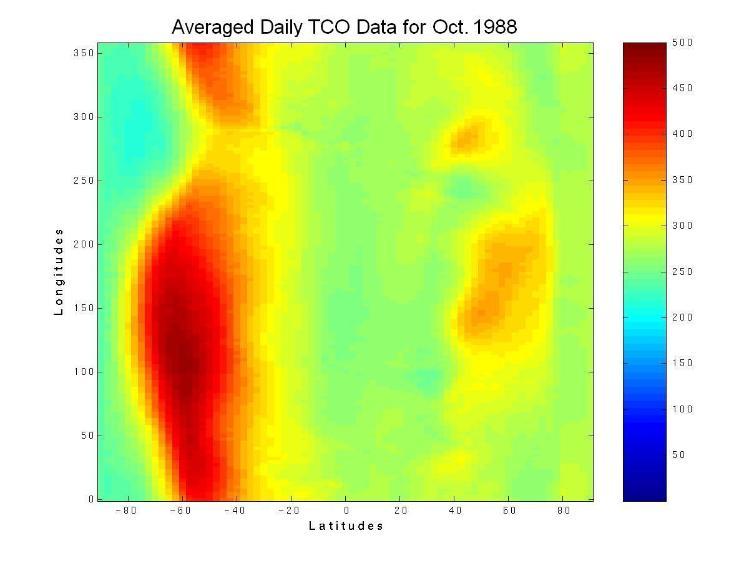
Figure 1. The spatial distribution of averaged daily total column ozone (TCO) data in Dobson Units (DU) for the month of October, 1988. The monthly averaged TCO data came from the British Atmospheric Data Centre (BADC).